ELISA (Enzyme-linked Immunosorbent Assay) has been a workhorse in biotech laboratories for decades. This well-established analytical technology is accessible and affordable, making it a staple across bioprocessing and diagnostic workflows. Yet, while it delivers reliable results, it also demands hours—sometimes overnight—to do so. This lag can hinder critical decisions and delay progress in fast-paced bioprocessing environments.
Bruno Oesch, a veteran biotech entrepreneur, is addressing this issue head-on. Through his company, Elionova, he aims to transform ELISA's static process into a real-time, dynamic system. His journey from academic prion research to building Prionics and now Elionova reflects a career driven by making science practically useful. With his latest breakthrough, he is transforming complex laboratory assays into agile tools for fast and reliable analytics.
This concept is discussed in greater detail in an episode of the Smart Biotech Scientist Podcast, hosted by David Brühlmann, founder of Brühlmann Consulting.
From Academic Curiosity to Biotech Entrepreneurship
Bruno Oesch's career began in academic research focused on prions. After a postdoctoral stint with Stan Prusiner, which contributed to Nobel-winning work, Bruno returned to Zurich and established his research group. During the height of mad cow disease, he and his team recognized the urgent need for a diagnostic test. Although their idea initially lacked support from industry and government bodies, they pressed on independently.
Their determination led to the creation of Prionics, where they successfully developed the first commercial BSE test. This achievement demonstrated the power of translating academic findings into real-world solutions. For Bruno, the thrill wasn't just solving scientific problems but applying those solutions practically. His entrepreneurial philosophy remains grounded in the principles of utility and accessibility.
Why ELISA Still Dominates Bioprocess Labs
First introduced in the 1970s, ELISA revolutionized protein analysis with a simple, colorimetric readout. It remains the go-to for many labs due to its:
- Familiar workflow
- Low material cost (plastic plates and reagents)
- Acceptable precision for most applications
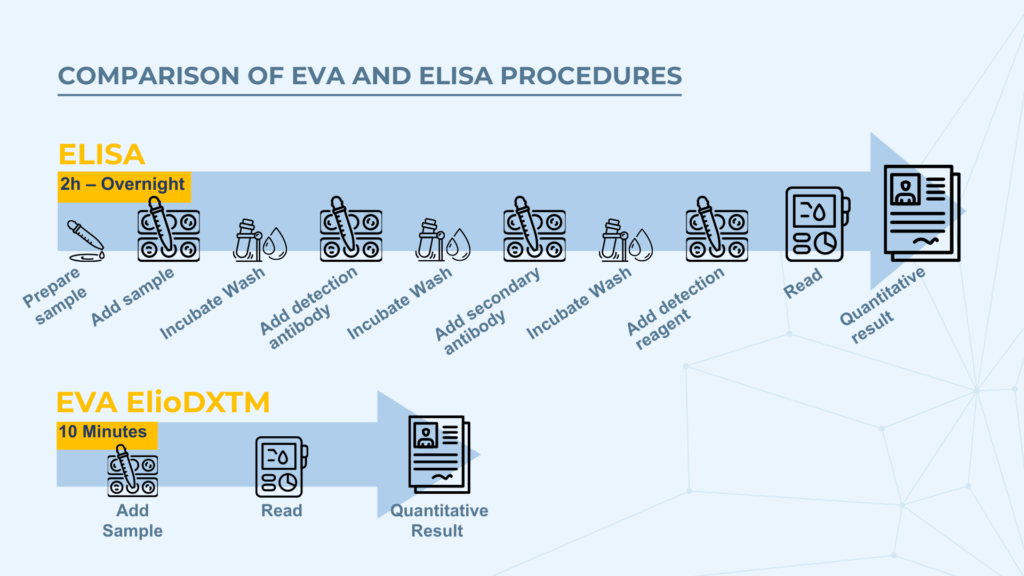
However, ELISA’s multi-step process—including binding, washing, and signal development—requires hours to complete. More advanced systems like Biacore (based on surface plasmon resonance) offer real-time sensitivity but at prohibitive costs.
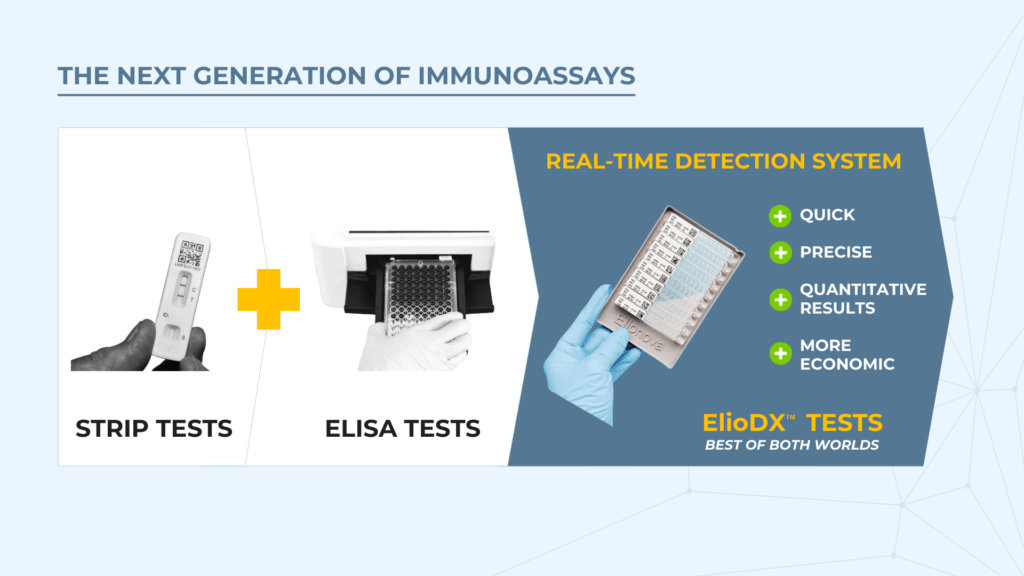
With its cost-effective plastic plates and straightforward setup, ELISA remains the gold standard despite its inefficiencies.
Introducing Real-Time Evanescence-Based Detection
Elionova's ElioDX technology builds on evanescence-based detection methods, which are historically known for their high sensitivity but limited practicality. Earlier implementations, such as Zeptasense, required expensive glass substrates and proprietary systems, pricing them out of routine use. ElioDX changes this.
The breakthrough lies in combining fluorescent detection with total internal reflection to create an evanescent field:
- A laser is directed into the bottom of the cavity, creating a shallow field of light
- Only fluorescent molecules within 200 nanometers of the surface are excited
- This means background molecules in the bulk solution do not interfere
In Bruno’s words, “We do biacore with fluorescents, and we do it on cheap materials. So we bring the Rolls-Royce down to the Volkswagen.”
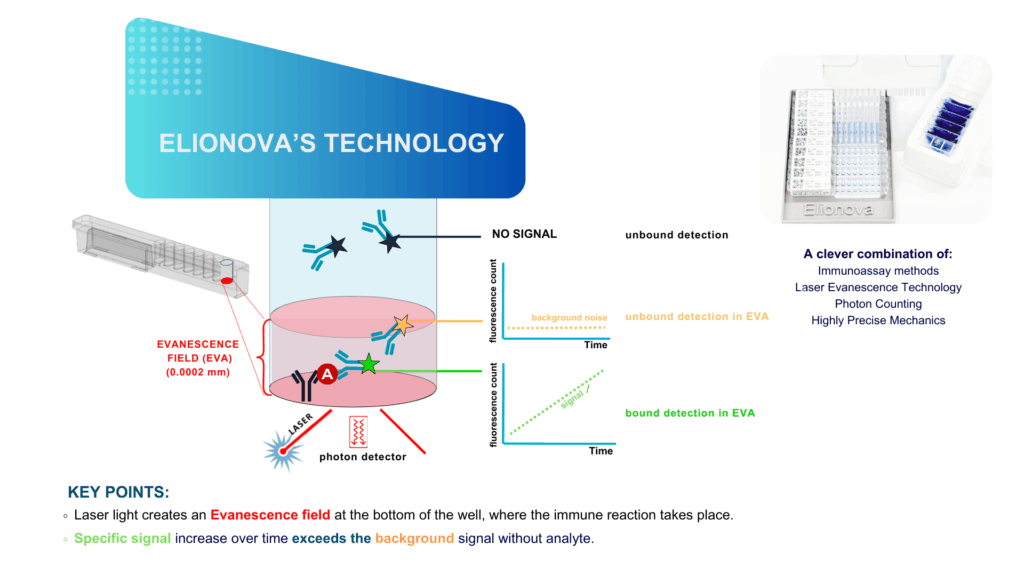
The reaction is monitored over time by measuring the fluorescence increase:
- No need for washing steps
- The slope of the fluorescence signal correlates with the analyte concentration
- Real-time measurement allows completion within 10 minutes
This approach accelerates the assay and automatically corrects for background signal by focusing solely on the signal change over time.
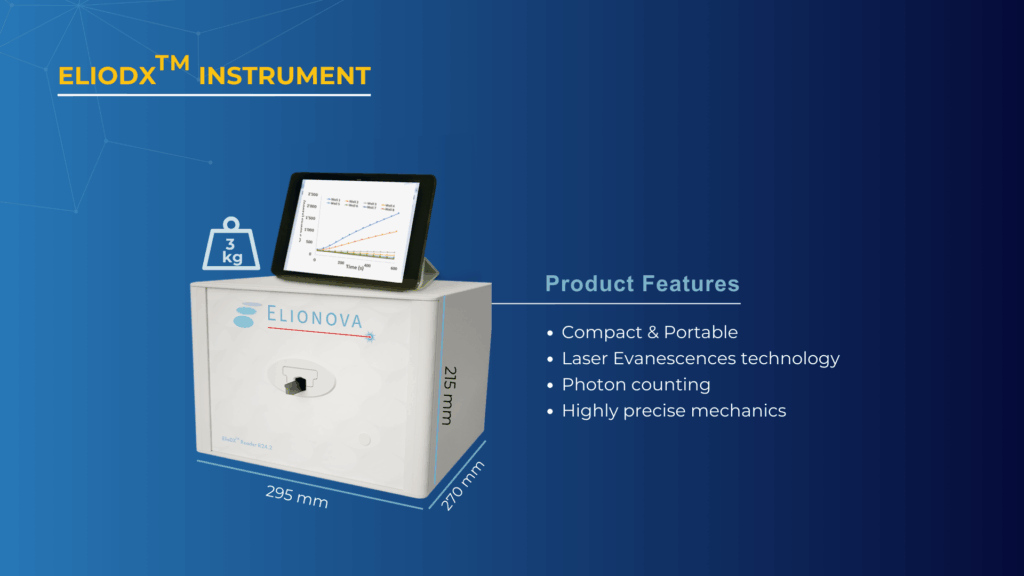
The Complete ElioDX Platform

The compact design makes the technology accessible across different laboratory environments. At just 3 kilograms and fitting easily on any benchtop, the ElioDX reader brings sophisticated evanescence detection to spaces where traditional analytical instruments would be impractical.
Elionova offers three tiers of assay solutions to match different user needs and expertise levels. Pre-made assays provide immediate deployment for common biomarkers like CRP, IL-6, and FSH, delivering results in 10 minutes with minimal setup. For specialized applications, customized assays can be developed in collaboration with Elionova's team. The DIY kits, including NeutrAvidin and Protein G options, give advanced users complete workflow control to adapt the platform for novel applications.

Tackling Matrix Effects and Complex Samples
Matrix effects can complicate protein detection in real-world samples, such as cell culture broth. ElioDX addresses this by calibrating the reference curve in the same matrix as the test samples. Additionally, dilutions can be used to ensure that results fall within a reliable range.
For example:
- CRP testing in plasma may require 10-fold, 100-fold, and 1,000-fold dilutions
- These dilutions are tested in the same cuvette to determine the appropriate range
- The device only requires 20 microlitres per cavity, optimizing sample use
This streamlined handling allows accurate quantification even in complex environments. Rapid diagnostics in these conditions empower scientists to make better, faster decisions.
Use Cases: Where Speed Beats Throughput
The ElioDX system currently processes one cuvette at a time. While unsuited for high-throughput operations, it excels in contexts where speed is more important than volume.
Examples include:
- Identifying peak harvest time during fermentation
- Determining residual protein post-chromatography
- Screening small sample sets in early-stage development
Traditional ELISA can still serve bulk analysis needs, but ElioDX complements it with agile, real-time feedback. For instance, a fermentation partner discovered that optimal protein production occurred after peak cell growth, not at the same time. This insight would have been missed using delayed ELISA methods.
Delivering Speed Without Compromising Sensitivity
One of ElioDX's most significant advantages is its speed. Results are available in 10 minutes, compared to hours or days. But what about sensitivity and accuracy?
Bruno confirms that ElioDX performs on par with or better than ELISA in terms of sensitivity, depending on the quality of the antibody. While technologies like Biacore offer unique label-free detection, they fall short in routine sensitivity and cost-effectiveness.
ElioDX bridges that gap with the following:
- ELISA-grade sensitivity
- Fluorescence-based precision
- Compatibility with existing antibodies and protocols
Additionally, adapting existing ELISA assays to ElioDX is straightforward. According to Bruno, transferring an assay takes roughly 10 days, using the same coating and incubation conditions. This reduces development overhead and accelerates implementation.
Overcoming Regulatory and Adoption Barriers
New technologies must meet regulatory standards before being adopted in clinical or GMP settings. ElioDX is being designed to comply with the following:
- GLP and GMP for regulated manufacturing
- IVD requirements for diagnostics
However, navigating the IVDR framework in Europe remains a hurdle due to limited capacity among notified bodies. As Bruno explains, larger companies are prioritized, making it more difficult for smaller players to gain traction.
As a result, Elionova is starting with applications for research use and with veterinary applications, where regulatory barriers are lower and early validation can build momentum for broader use.
The App Store for Assays: Democratising Development
A standout feature of Elionova's business model is openness. Users can create their assays and share them with the broader community.
This vision includes:
- A digital assay store (ALI-E store), an open assay marketplace
- Opportunities for users to publish, license, and sell their custom assays
- Community-driven troubleshooting and innovation
This community-driven model democratizes assay development. By supporting assay developers rather than locking them into proprietary systems, Elionova fosters flexibility and collaboration. The only requirement is purchasing the base materials and instruments from Elionova.
This strategy allows rapid expansion of application areas without requiring Bruno's team to build everything in-house. It also mirrors the ELISA ecosystem, where many labs develop and share protocols informally.
Versatile Applications and Streamlined Workflow
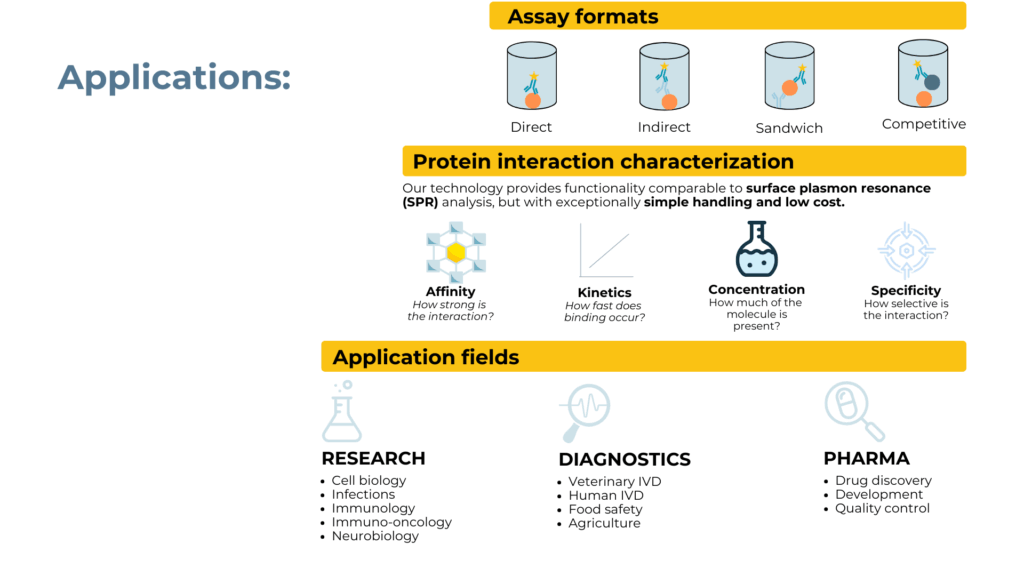
The versatility of the ElioDX platform extends across multiple assay formats and application domains. Whether researchers need direct, indirect, sandwich, or competitive immunoassays, the evanescence-based detection adapts to different molecular architectures. This flexibility, combined with protein interaction characterization capabilities comparable to surface plasmon resonance, positions ElioDX as a comprehensive solution for research, diagnostics, and pharmaceutical quality control.
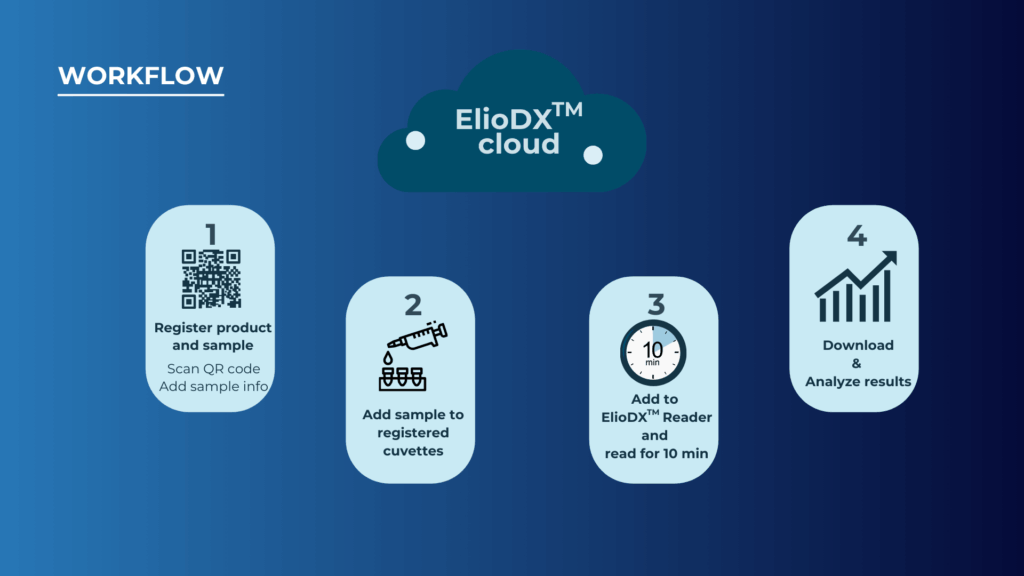
The user experience reflects this emphasis on simplicity and integration. The entire workflow—from sample registration through data analysis—is designed around modern laboratory data management practices. Users begin by scanning QR codes to register their samples in the cloud platform, add samples to pre-loaded cuvettes, insert them into the reader for 10-minute analysis, then download and analyze results through the integrated digital platform.
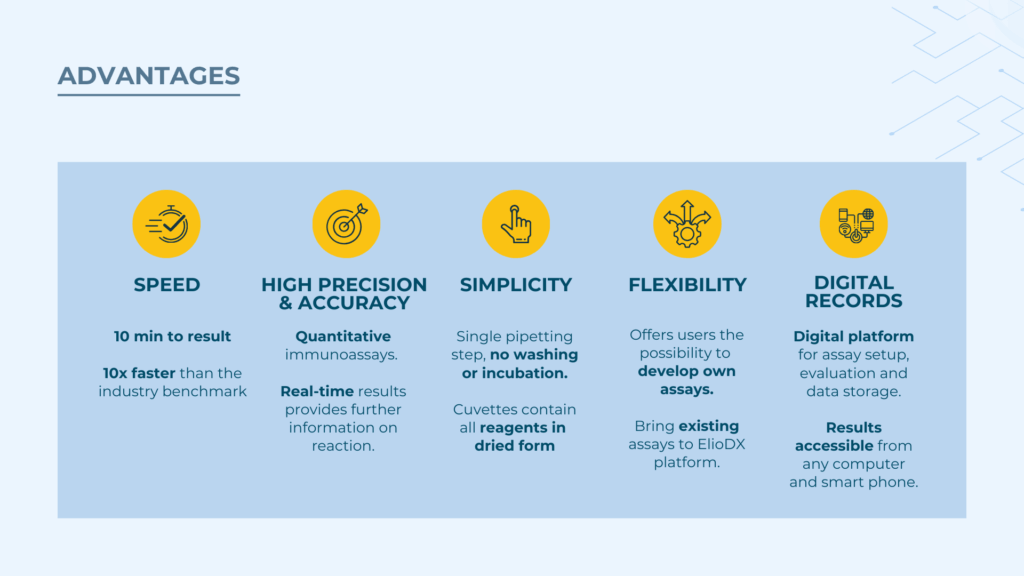
The combined advantages create a compelling value proposition that Bruno believes will drive adoption despite the biotech industry's inherent conservatism. ElioDX delivers results 10 times faster than traditional methods, maintains high precision with quantitative real-time data, simplifies workflows to a single pipetting step, offers flexible assay development capabilities, and provides comprehensive digital data management—all while reducing overall costs compared to traditional approaches.
The Vision for Real-Time Diagnostics and Point-of-Care Testing
Bruno envisions a world where diagnostics are fast, portable, and decentralized. While early adoption will take place in research and veterinary labs, the ultimate goal is to bring testing to:
- Physicians’ offices
- Emergency departments
- Field clinics
Time-sensitive conditions like sepsis and a heart attack require rapid intervention. If clinicians can test biomarkers at the point of care and act immediately, patient outcomes could improve dramatically.
For this to happen, Elionova must secure:
- IVD certification for instruments and assays
- Clinical validation in target applications
- Partnerships with established diagnostic providers
Bruno is open to collaborating with partners to accelerate the process, but he is fully prepared to pursue it independently if needed, as he successfully did with Prionics.
Entrepreneurial Wisdom from a Serial Innovator
Bruno Oesch has walked the whole path from research to commercialization. His advice to aspiring biotech entrepreneurs is grounded in experience:
- Be convinced: You must believe in your technology, even if others don't
- Be persistent: Push through rejection and keep testing your value proposition
- Know your customer: Identify a clear use case and communicate it effectively
- Be strategic: Have a hook. For BSE, they challenged official narratives by proving hidden risks.
Success requires a balance of stubborn vision and open-minded validation. Bruno highlights that even with strong resistance, change is possible when the solution is too good to ignore.
Breaking Through Conservatism in Biotech
The biotech industry is notoriously conservative, favoring incremental improvement over radical innovation. Bruno challenges this mindset by offering a leap forward that simplifies workflows, reduces time, and empowers better decision-making.
His secret weapon? Exposure. Labs that try ElioDX don't want to go back. Once you’ve experienced 10-minute, no-wash immunoassays, returning to slower, manual methods is hard. Elionova allows the technology to speak for itself by giving labs free trials. As more users experience its benefits, word of mouth and community building will take care of the rest.
Final Thoughts: A New Standard for Immunoassays
Elionova's ElioDX platform represents a pivotal shift in immunoassay technology. It retains the trusted sensitivity of ELISA while eliminating its most frustrating delays. A user-friendly setup, compatibility with existing protocols, and rapid feedback offer a much-needed tool for modern bioprocessing.
Real-time analytics are no longer a luxury. They are becoming essential for staying competitive and responsive. Whether you're managing upstream fermentation, downstream purification, or quality control, the ability to act on data immediately can transform your outcomes.
This is more than a technological improvement. It's a mindset shift: from static measurement to dynamic monitoring, from delayed insights to instant decisions. This might be your next essential tool if you're ready to simplify, speed up, and smarten your biotech workflows.
About Bruno Oesch
Bruno Oesch is a biochemist (ETH Zurich) and molecular biologist (PhD University of Zurich). Having worked on prions for over a decade, he founded Prionics AG, the first company to market a BSE test and other veterinary tests, which was later sold to ThermoFisher.
He further established and managed several companies in the biotech sector. The latest endeavour is Elionova AG, revolutionizing immunoassays (real-time one-step assay with quantitative results in 10 min).
Connect with Bruno Oesch on LinkedIn.
David Brühlmann is a strategic advisor who helps C-level biotech leaders reduce development and manufacturing costs to make life-saving therapies accessible to more patients worldwide.
He is also a biotech technology innovation coach, technology transfer leader, and host of the Smart Biotech Scientist podcast—the go-to podcast for biotech scientists who want to master biopharma CMC development and biomanufacturing.
Hear It From The Horse’s Mouth
Want to listen to the full interview? Go to Smart Biotech Scientist Podcast.
Want to hear more? Do visit the podcast page and check out other episodes.
Do you wish to simplify your biologics drug development project? Contact Us

